The Thora Tech Academy
We offer you both individually tailored consulting and support in the development of medical devices, especially in the area of regulatory requirements - as well as the necessary basic knowledge in the form of our certificate training courses.
Individual advice and support
Quality management systems for Manufacturers of Medical Devices (EN ISO 13485)
Implementation of the Medical Devices Ordinance
(MDR)
Risk management for Medical Devices
(EN ISO 14971)
Usability-oriented development process (EN ISO 62366-1)
Electrical safety of medical devices (EN 60601-1)
Programmable Electrical Medical Systems (PEMS)
Software Lifecycle
(EN 62304)
Technical documentation for medical devices
... in expert discussions we show you the way from the idea to the product!
We work with you to develop an individual concept for the realization of your product specifications.
We help you to observe and comply with the regulatory and normative requirements.
We support you in the implementation of the technical design and the creation of the technical documentation.
We would be happy to provide you with support and handle the overall product development.
Certificate training course
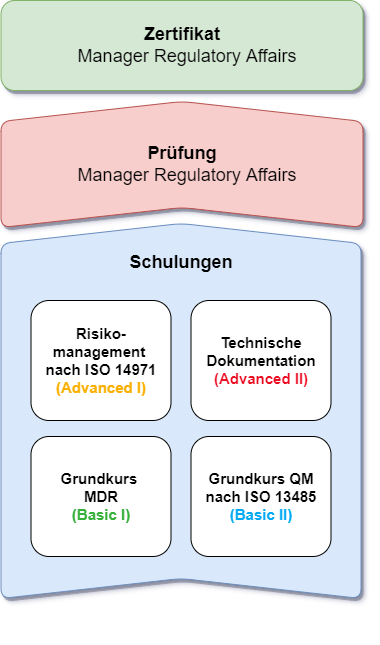
Our training courses
Complete all four courses and receive your own certificate as a
Regulatory Affairs Manager.
Basic I
Basic course Medical Device Regulation MDR
Duration: 1 Day
Basic II
Quality management systems for manufacturers according to ISO 13485
Duration: 1 Day
Advanced I
Risk management for medical devices according to ISO 14971
Duration: 2 Days
Advanced II
Technical documentation for medical devices
Duration: 1 Day
Frequently asked questions
The exam takes about 30 minutes. The examination fee is 70 euros per examinee.
Yes! In order for a course to take place, at least five participants must be registered per course.
The training courses can take place as in-house training courses both online or in presence, if the hygiene measures ensure the safety.
An in-house training course is an instrument of company training in the context of personnel development. Employees receive a customized in-house course taught by external instructors.
The trainings are held by our employee / consultant Prof. Dr. Michael Scholtes.
Professor Dr Scholtes works full-time at the Technischen Hochschule Mittelhessen, where he holds the professorship for Digital Medicine with a focus on Regulatory Affairs.
Contact us
You would like to request a consultation / training without obligation or have general questions? Please do not hesitate to contact us!

